During the trial, doses of the trial drug proven to be safe (tested during Phase I trials) will be given to you over a specified time period, fully funded by the sponsor3. Data assessing the impact of the drug will be collected frequently and so it is likely that you will have to live in a clinical trial facility or regularly visit the site, benefiting from increased medical attention as a result.
Clinical Trials in Hong Kong
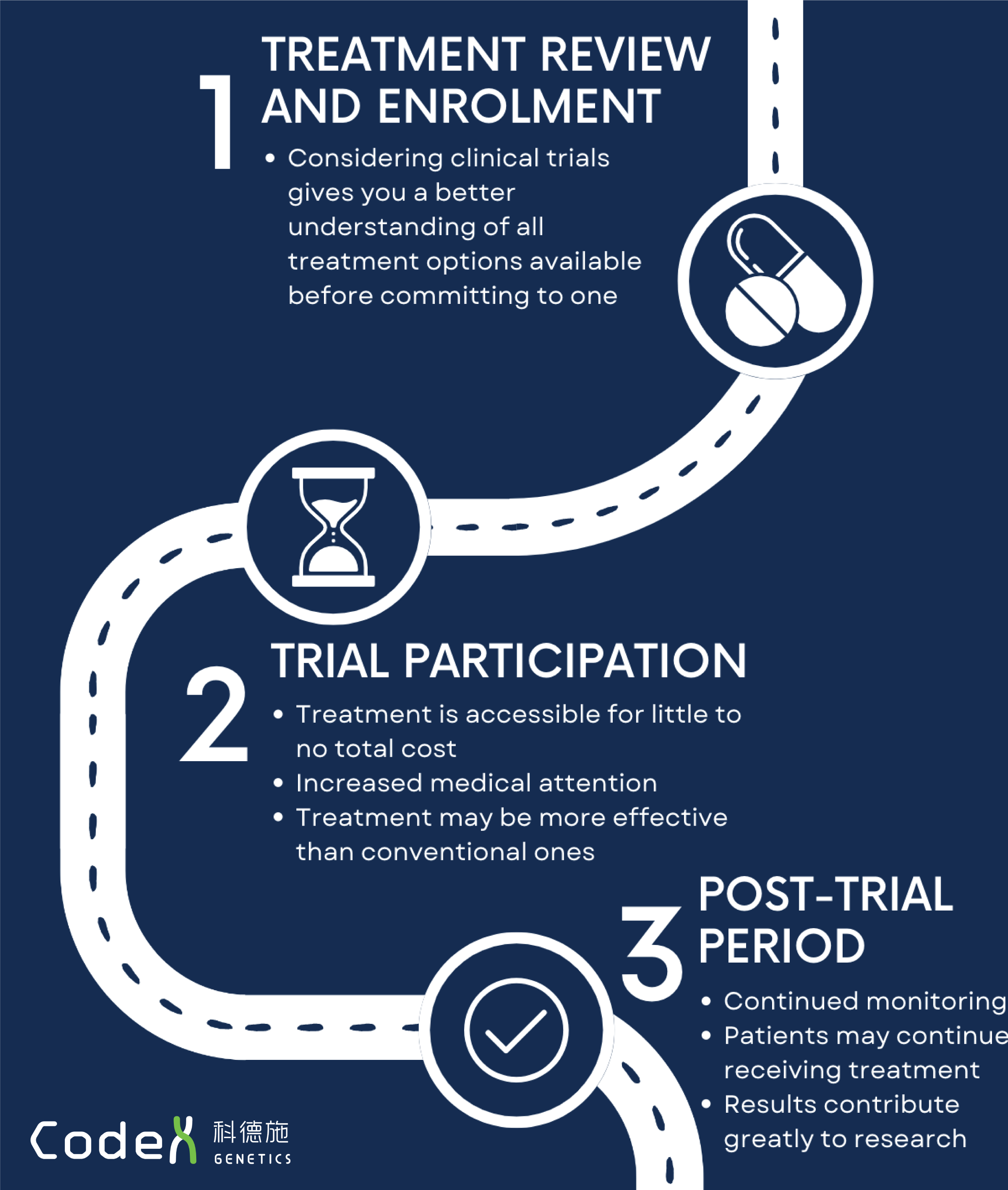
Clinical trials are performed to research the effects of newly developed drugs in patients but importantly also aim to treat participants in the process. Hong Kong currently provides over 230 Phase II-IV trials1 that have been proven safe and effective for patients with diverse conditions ranging from cancer to neurodegenerative diseases. With such a wide range of trials available, it is highly likely that there is a trial perfectly suited for every patient. Certain trial drugs may align more closely with the specific needs of an individual, offering better performance and outcomes compared to conventional treatments. Therefore clinical trials should be seen as a valuable treatment option from an early stage, not only for cases with no other options left. Understanding not only the benefits that you can gain from participation but also the whole process from enrolment to completion is vital for selecting the best trial for you when discussing with your doctor.
Signing up to a trial
Although there are many trials in Hong Kong actively recruiting patients, the conditions of a pre-defined eligibility criteria must be met before you are allowed to participate. As a result of this, having access to comprehensive medical information for example from our CoGenesis® genetic tests is crucial in guiding discussions with your doctor during a treatment review and identifying the trials most suitable for you.
If relevant trials are identified and eligibility criteria are met, a doctor will then be able to provide in-depth details surrounding the entire process of the trial, the phase of the trial, any potential risks, how your safety is protected, your right to drop-out, coverage of costs and more2. Ultimately, this discussion with your doctor is an important step in allowing you to be fully informed before deciding whether to commit to a trial as a treatment.
The trial itself
During the trial, doses of the trial drug proven to be safe (tested during Phase I trials) will be given to you over a specified time period, fully funded by the sponsor3. Data assessing the impact of the drug will be collected frequently and so it is likely that you will have to live in a clinical trial facility or regularly visit the site, benefiting from increased medical attention as a result.
On top of the increased monitoring benefits, all costs pertaining to the trial and any extra medical expenses are covered by the sponsor or insurance3, allowing you to access cutting-edge treatments and contribute towards life-changing medical research without significant financial burden.
However, it is important to note that the trial process is not always smooth sailing. After you take the trial drug, it is possible for you to experience adverse side-effects that were previously unknown. In the event of this occurring, the increased medical attention mentioned earlier is vital to ensure you are rapidly attended to, preventing any side effects from severely affecting your health. Should the negatives of participation like this outweigh the positives, you have the right to drop-out of the trial at any time and search for alternative treatment options.
However your overall well-being is the top priority during a clinical trial, ensuring that you have a positive experience whilst receiving effective treatment throughout the entire process.
Following the trial
In many cases, monitoring will continue even following completion to investigate the long-term effects of the drug. Importantly, trial completion does not mean that you will cease to receive the trial drug as in most cases, treatment can continue should you have benefited significantly from the trial3. In these cases the trial drugs can become a first-line treatment for you, proving how crucial clinical trial participation is in acting as a gateway to accessing life-changing treatment.